Deciphering the Electrostatic Nature of Hydrogen Bonds in Nanoconfined Water Using a Dipole-in-E-Field Model
Key Ideas
- Hydrogen bonds (HBs) play a crucial role in altering the properties of systems like water, ices, hydrogels, proteins, and DNA molecules by forming extensive networks over long ranges.
- A new dipole-in-E-field approach is introduced to quantitatively describe HBs, considering the electric dipole moment of the donor-hydrogen pair interacting with the induced electric field from the acceptor.
- This model provides a more universal and precisely quantifiable way to understand HB strength and directionality, offering insights into complex HB networks in systems like nanoconfined water.
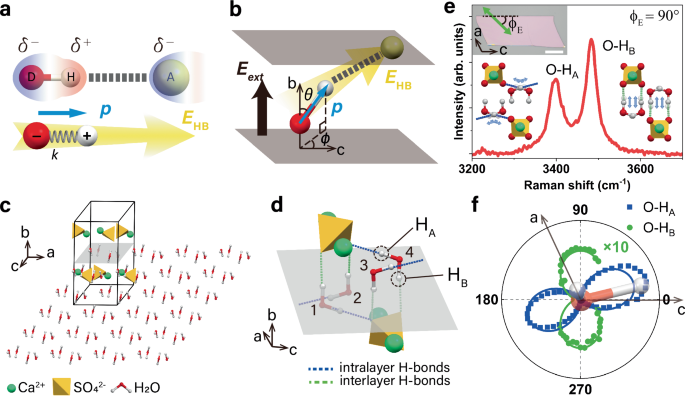
- The study utilizes naturally occurring hydrogen-bonded heterostructure gypsum to demonstrate the application of the dipole-in-E-field approximation for HB analysis in nanoconfined water.
Hydrogen bonds (HBs) are crucial intermolecular interactions with unique properties that significantly influence the behavior of various systems such as water, ices, hydrogels, proteins, and DNA molecules. Despite their importance, analyzing HBs in complex systems has been challenging due to their directional and electrostatic nature. In a recent study, researchers introduced a novel approach to understand HBs by conceptualizing them as an electric dipole moment interacting with an electric field induced by the acceptor group. This dipole-in-E-field model offers a more precise and universal way to quantify HB strength and directionality, enabling predictions about the properties of systems with complex HB networks. By using this model, the researchers were able to analyze HBs in nanoconfined water within a hydrogen-bonded heterostructure gypsum system. The study demonstrates how the new approach can provide insights into the electrostatic nature of HBs and the effects of external electric fields on these interactions. This research not only enhances our understanding of HBs but also offers a practical method to predict and analyze hydrogen bonding in various systems, paving the way for advancements in this field.
Topics
Power
Intermolecular Interactions
Electrostatic Model
Water Properties
HB Networks
Quantitative Description
Nanoconfined Water
Latest News