Advancements in Catalyst Technology for Efficient Hydrogen Production and Methane Oxidation
Key Ideas
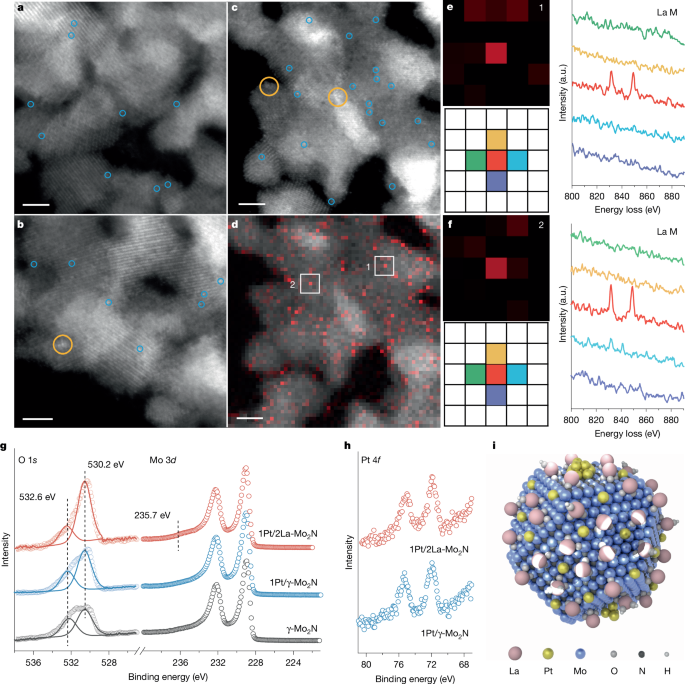
- Recent studies have focused on developing catalysts with atomically dispersed metal centers to enhance catalytic performance.
- Research has shown that catalysts like Pt/α-MoC and Ni/α-MoC demonstrate efficient low-temperature hydrogen production from water and methanol.
- Innovative approaches, such as using fully exposed cluster catalysts and single-atom catalysts, have shown promise in hydrogen production and methane oxidation.
- The use of alloy catalysts, metal-support interfaces, and stable catalyst structures like Pd@CeO2 subunits have significantly improved catalytic activity for various reactions.
The article discusses the significant progress made in catalyst technology for various catalytic reactions related to hydrogen production and methane oxidation. Researchers have been focusing on developing advanced catalysts with atomically dispersed metal centers to enhance catalytic performance. Studies involving catalysts like Pt/α-MoC and Ni/α-MoC have demonstrated efficient low-temperature hydrogen production from water and methanol. Innovative approaches such as fully exposed cluster catalysts and single-atom catalysis have shown promising results in improving hydrogen production and methane oxidation processes. Additionally, the use of alloy catalysts, metal-support interfaces, and stable catalyst structures like Pd@CeO2 subunits have significantly enhanced catalytic activity for reactions such as methane combustion and carbon monoxide oxidation. These advancements in catalyst technology are crucial for achieving more efficient and sustainable processes in hydrogen production and methane oxidation.
Topics
Production
Catalysts
Nanotechnology
Methanol
Carbon Monoxide
Oxidation
Water-gas Shift
Nitrogen Heterocycles
Methane Combustion
Latest News