Innovative PtRuNi-Ox Catalysts for Efficient Hydrogen Production in Real Wastewater Environments
Key Ideas
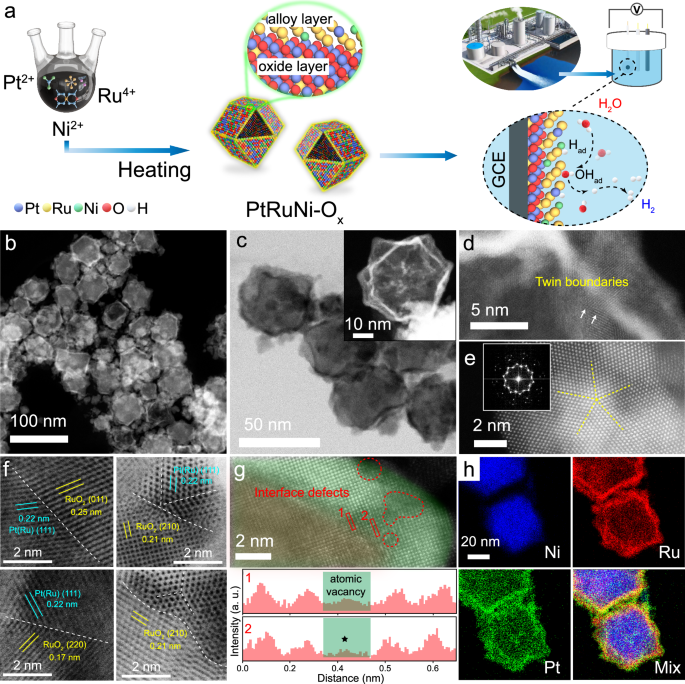
- Development of PtRuNi-Ox catalysts with twin boundary distortion structures enhances catalytic efficiency and stability in hydrogen production.
- The novel catalyst exhibits excellent HER activity in alkaline freshwater and chemical wastewater, surpassing commercial Pt/C.
- PtRuNi-Ox requires low overpotentials in alkaline chemical wastewater and seawater electrolytes, showing potential for real wastewater treatment.
- Materials synthesis and characterization reveal the unique structure of PtRuNi-Ox hollow nanocages with high surface area and abundant active sites for catalytic reactions.
Hydrogen, a zero-emission energy alternative, can be produced sustainably from renewable sources like wastewater. The article discusses the challenges of using catalysts in wastewater environments and the importance of developing stable catalyst materials. Ruthenium and its compounds show promise in water dissociation but face oxidation limitations. PtRuNi-Ox catalysts, designed with twin boundary distortion structures, demonstrate exceptional catalytic efficiency in hydrogen evolution reactions (HER). These nanocages provide numerous active sites and elevated surface energy, enhancing catalytic reactions. The article details the synthesis and characterization of PtRuNi-Ox catalysts, highlighting their hollow nanocage structure with twin boundaries, which contribute to their stability and efficiency. Results show that PtRuNi-Ox outperforms commercial Pt/C in HER activity and maintains stability after 40,000 cycles. The catalyst also shows potential in alkaline freshwater, chemical wastewater, and seawater electrolytes, indicating promising applications in real wastewater treatment for environmental protection.
Topics
Production
Renewable Energy
Sustainability
Catalysts
Electrochemistry
Catalytic Materials
Water Treatment
Synthesis
Nanocages
Latest News