Advancements in Electrochemical Ammonia Synthesis: A Comprehensive Review
Key Ideas
- Researchers have made significant progress in electrochemical methods for ammonia synthesis, offering a potential COx-free hydrogen production route for fuel cell applications.
- Studies have focused on enhancing the efficiency, stability, and selectivity of lithium-mediated electrochemical nitrogen reduction processes to improve ammonia generation.
- Novel approaches, such as using calcium as a mediator, have shown promise in increasing the rate and selectivity of ammonia production through electrochemical processes.
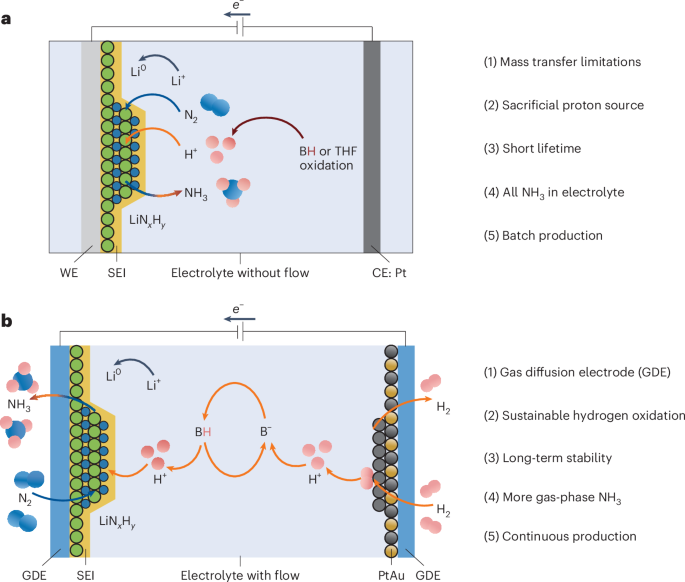
- Efforts to optimize electrode materials, electrolyte composition, and proton shuttle mechanisms have played a crucial role in advancing the field of electrochemical ammonia synthesis.
The article delves into the latest advancements in electrochemical ammonia synthesis, showcasing a shift towards sustainable and efficient methods for ammonia production. Researchers have been exploring electrochemical routes for the synthesis of ammonia as a means to achieve COx-free hydrogen production suitable for fuel cell applications. Studies have focused on enhancing the efficiency, stability, and selectivity of electrochemical nitrogen reduction processes. Novel approaches, such as utilizing calcium as a mediator, have demonstrated the potential to improve the rate and selectivity of ammonia generation. Electrode material optimization, electrolyte design, and proton shuttle mechanisms have been key areas of research to enhance the performance of electrochemical processes. The review highlights the progress in increasing current density, surface area utilization, and selectivity in ammonia synthesis through innovative electrochemical techniques. By incorporating advancements in electrochemistry and catalysis, researchers aim to develop sustainable and scalable methods for ammonia production, paving the way for a cleaner energy future.