Revolutionizing Clean Energy: Oregon State University's Breakthrough in Hydrogen Production
Key Ideas
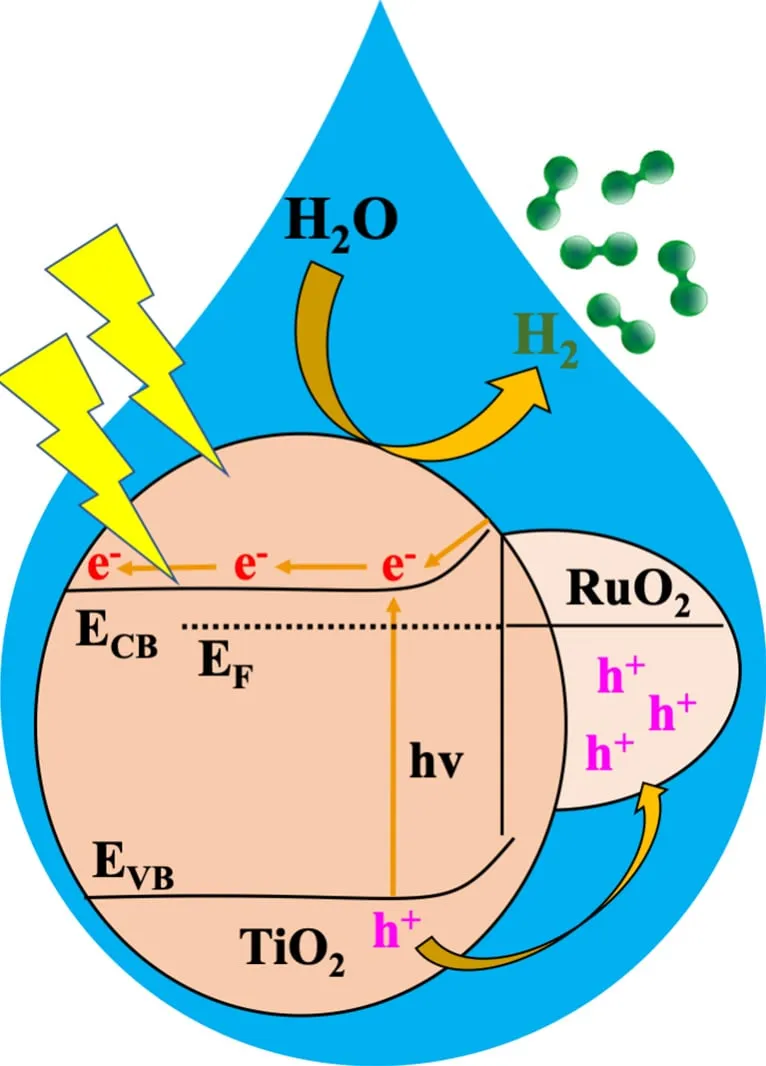
- Researchers at Oregon State University have developed a Metal-Organic Framework (MOF) catalyst, RTTA-1, capable of efficiently producing hydrogen from sunlight and water.
- The catalyst showed outstanding performance, producing over 10,700 micromoles of hydrogen per gram in one hour, utilizing just 10% of received light, offering a promising solution for clean energy production.
- MOFs, known for their high surface area and tunable properties, have a wide range of applications including gas storage, separation, catalysis, and pollution control, making them crucial for advancing clean energy technologies.
A team led by Kyriakos Stylianou at Oregon State University has made significant progress in clean energy production by developing a catalyst named RTTA-1, derived from Metal-Organic Frameworks (MOFs). This catalyst efficiently splits water into hydrogen when exposed to sunlight, offering a sustainable energy solution for various applications, including powering cars and industrial processes. The team's research highlighted the exceptional performance of RTTA-1, showcasing its ability to produce hydrogen rapidly and effectively, with minimal light utilization. Metal-Organic Frameworks, such as MOFs, play a crucial role in advancing clean energy technologies due to their unique properties like high surface area and customizable characteristics, allowing for applications in gas storage, separation, catalysis, and pollution control. Overall, this breakthrough by Oregon State University researchers paves the way for cleaner hydrogen production methods and contributes to the global efforts towards reducing greenhouse gas emissions.