Chinese Breakthrough in Green Hydrogen Production Catalysts
Key Ideas
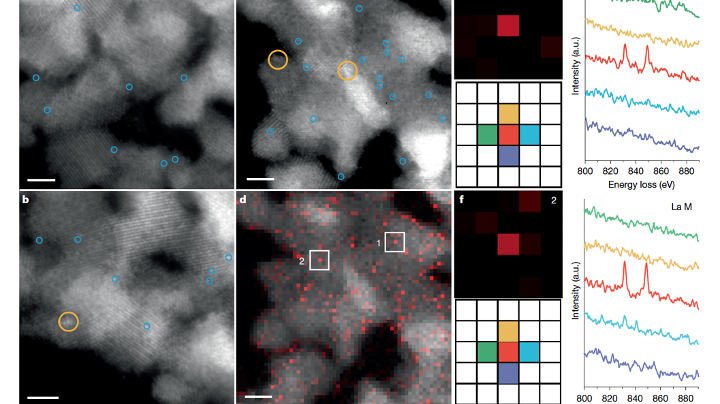
- Chinese scientists achieved a catalyst stability of over 1,000 hours for methanol reforming, a key step for large-scale hydrogen production.
- The rare earth oxide nano-coating on platinum catalysts enhanced stability, with a turnover number of 15 million hydrogen molecules per platinum atom.
- The breakthrough could lead to low-cost, large-scale hydrogen production, crucial for global energy transition towards carbon neutrality.
- Additionally, the team developed a method for carbon dioxide-free hydrogen production from ethanol by fixing carbon atoms into acetic acid at mild temperatures.
Chinese scientists have made significant strides in catalytic hydrogen production, achieving a catalyst stability of over 1,000 hours for methanol reforming. The development of a rare earth oxide nano-coating on platinum-based catalysts has significantly increased stability while maintaining high activity levels. This breakthrough, published in Nature, is a crucial step towards large-scale hydrogen production. The research is seen as a key advancement in the quest for green and efficient hydrogen production technologies, essential for global energy transition towards carbon neutrality. The team's success in achieving carbon dioxide-free hydrogen production from ethanol at mild temperatures further demonstrates their commitment to environmentally friendly and sustainable energy solutions. While the high cost of precious metals remains a challenge, the focus now turns to cost reduction to facilitate industrial application. The potential impact of this research extends beyond hydrogen production, with implications for various sectors like marine transportation and electrical energy conversion.