Innovative Catalyst Design for Green Hydrogen Production from Bio-Ethanol Reforming
Key Ideas
- Utilizing a novel catalyst design involving single atom catalysts and adjacent nanoclusters has shown promise in enhancing the efficiency of bio-ethanol reforming for green hydrogen production.
- The newly synthesized xNi/Mo2TiAlC2 catalyst series exhibited outstanding activity in steam reforming of bio-ethanol, achieving a hydrogen utilization efficiency of 67.8% at 550°C.
- The synergy between single atom and nanocluster components in the catalyst structure was found to significantly enhance ethanol adsorption, activation, and catalytic performance, leading to a 700% increase in hydrogen yield per Ni atom compared to conventional catalysts.
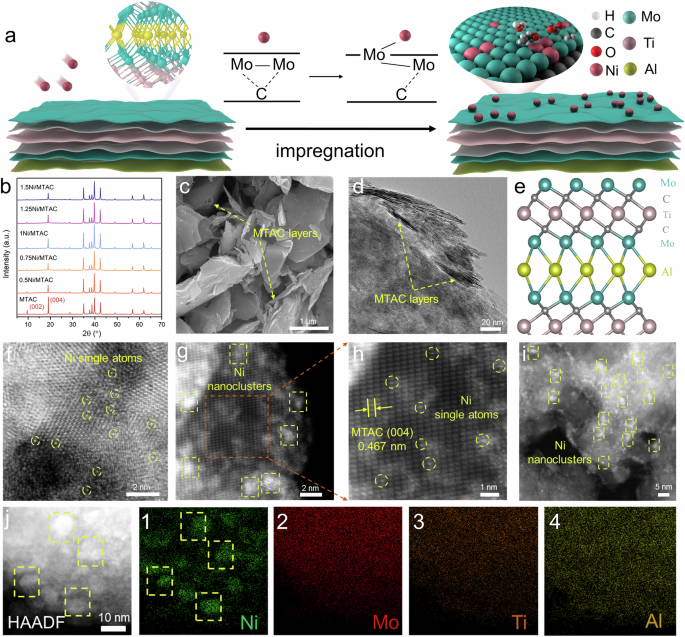
- Characterization studies revealed the successful synthesis of a range of xNi/MTAC catalysts and demonstrated the structural configuration of the catalysts through XRD patterns, SEM, TEM images, and HAADF-STEM analysis.
Hydrogen, as a clean and high-energy-density fuel, is gaining traction as a promising alternative to fossil fuels for a sustainable energy future. One crucial aspect of hydrogen production is the steam reforming of bio-ethanol (SRE), which offers a green pathway for hydrogen generation. While nickel has been a standard metal catalyst for SRE due to its efficiency in bond dissociation and cost-effectiveness, challenges like deactivation through sintering and carbon deposition have persisted. To address these limitations, recent research has focused on innovative catalyst designs, notably single-atom catalysts (SACs) and their synergistic combination with nanoclusters. The article discusses a study that successfully synthesized a series of xNi/Mo2TiAlC2 catalysts to enhance the efficiency of ethanol reforming for hydrogen production.
The study demonstrated that the 1Ni/MTAC catalyst from the series exhibited exceptional SRE activity with a hydrogen utilization efficiency of 67.8% at 550°C, remaining stable over 120 hours without deactivation. This catalyst design, incorporating a combination of single atoms and adjacent nanoclusters, showed promising results in promoting ethanol adsorption, activation, and overall catalytic performance. Notably, the hydrogen yield per Ni atom increased by 700% compared to traditional catalysts, underscoring the effectiveness of the new catalyst configuration.
Characterization of the Ni/MTAC catalysts involved various analyses, including XRD patterns, SEM, TEM images, and HAADF-STEM analysis, illustrating the structural details and composition of the synthesized catalysts. The findings highlight the significance of exploring advanced catalyst designs, such as SACs and nanocluster-SAC combinations, in optimizing bio-ethanol reforming for efficient green hydrogen production. The research provides valuable insights into the potential of tailored catalyst structures for enhancing the performance and stability of hydrogen production processes.
Topics
Production
Renewable Energy
Catalysts
Catalytic Efficiency
Nanoclusters
Atom Utilization
Ethanol Reforming
Latest News