Unveiling the Mystery of Water Wires through Computational Spectroscopy
Key Ideas
- Computational spectroscopy uncovers a potential signature of water wires in water and ice through UV-to-visible light absorption measurements.
- Water wires, composed of strongly hydrogen-bonded water molecules, play a crucial role in proton transfer, electrical conduction, and biological processes.
- State-of-the-art techniques, including many-body perturbation theory and path-integral molecular dynamics, validate the presence and impact of water wires.
- The study provides a foundation for future experiments to explore the length, stability, and behavior of water wires under varying conditions using UV-visible spectroscopy.
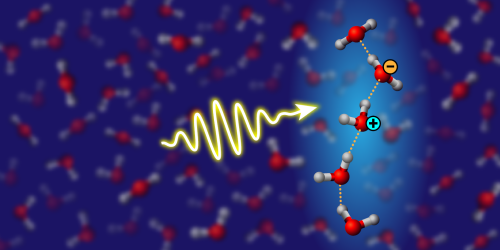
A recent theoretical study by Fujie Tang and colleagues has delved into the realm of water wires, linear structures of more strongly hydrogen-bonded water molecules, which have been elusive in direct observations. Through rigorous electronic-structure calculations and molecular-dynamics simulations, the researchers propose a method to detect water wires in bulk water and ice by analyzing light absorption in the UV-to-visible range. The study sheds light on how water wires facilitate proton transfer, impacting electrical conduction in ice, acid–base chemistry in water, and biological processes. By combining simulations and calculations, the researchers identify a spectroscopic fingerprint of water wires in the UV absorption spectrum, linked to collective excitations and charge-transfer excitons. The computational techniques utilized in the study, including artificial intelligence to enhance quantum simulations, provide accurate descriptions of light–matter interactions in hydrogen-bonded systems. The results show that the intensity of the spectroscopic peak is highest in proton-ordered ice, where water wires are stable and extended, and weakest in liquid water, where the wires dynamically form and break. Future experiments could focus on monitoring spectral features to understand the behavior of water wires under different conditions, offering insights into proton transfer mechanisms in various chemical and biological processes.
Topics
Fuel Cells
Spectroscopy
Molecular Dynamics
Water Wires
Proton Transfer
Optical Spectra
Charge-transfer Exciton
Experimental Characterization
Hydrogen Bonds
Latest News