Revolutionizing Hydrogen Peroxide Production: A Sustainable and Efficient Breakthrough
Key Ideas
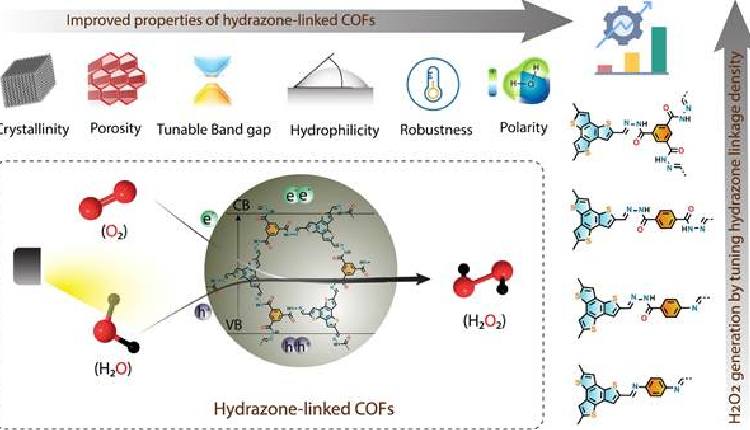
- Researchers at S. N. Bose National Centre for Basic Sciences in Kolkata have developed a groundbreaking method to synthesize hydrogen peroxide using covalent organic frameworks (COFs) with enhanced water affinity and photocatalytic properties.
- The new approach offers a more environmentally friendly and energy-efficient alternative to the current industrial method, reducing hazardous by-products and energy consumption significantly.
- The hydrazone-linked COFs showed exceptional performance in H2O2 generation under both blue LED and sunlight irradiation, paving the way for a sustainable production process with minimal environmental impact.
- This innovation not only enhances H2O2 production but also provides a pathway for technology transfer from laboratory research to industrial application, promising a cleaner and more sustainable future for chemical synthesis and disinfection industries.
Researchers at the S. N. Bose National Centre for Basic Sciences in Kolkata, India, have made a significant breakthrough in hydrogen peroxide (H2O2) production. Hydrogen peroxide is a crucial chemical used in various industries, including disinfection, paper bleaching, and fuel cells. The traditional method of producing H2O2 using the anthraquinone oxidation process is energy-intensive, expensive, and generates hazardous by-products. In their study, the researchers designed covalent organic frameworks (COFs) with enhanced water affinity to facilitate the photocatalytic generation of H2O2. These COFs provide abundant docking sites for water and oxygen, promoting water oxidation and oxygen reduction reactions necessary for H2O2 production. The hydrazone-linked COFs demonstrated exceptional performance under blue LED and sunlight irradiation, surpassing many organic photocatalysts. This innovative approach not only improves H2O2 production efficiency but also ensures minimal environmental impact. By utilizing a water-benzyl alcohol solution, the method prevents H2O2 degradation and offers a potential pathway for continuous flow production in a sustainable manner. The researchers emphasize the technology transfer potential of their findings, highlighting the prospect of transitioning from laboratory-scale production to industrial applications for the benefit of society and the environment.